CHEMISTRY 1 – CALORIMETRY LAB NAME HOUR
OBJECTIVE
Determine the energy content (in calories) of a food product
Materials:
Walnut, stopper with wire, pop can, temperature probe/computer, ring stand, ceramic centered wire gauze, clamps
PROCEDURE/RATIONALE
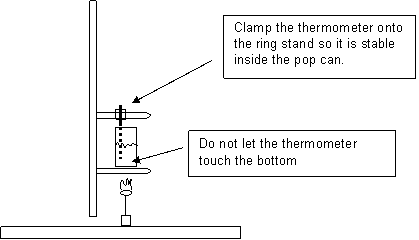
Burn the food, and use the heat generated to heat a measured quantity of water using the temperature probe from the computer. Run the Logger Pro file “Calorimetry Lab” in the Chemistry 1 folder on the desktop.
Measure the temperature of the water to determine the temperature change.
Use the equation Q = s x m x DT to determine the energy content of the food.
LAB DATA PACKAGE DATA
Mass of food g Type of food
Volume of water in calorimeter mL Serving size g
Initial
Temperature of water oC Calories per serving
Final Temperature of water oC (Note: Calories listed in nutritional info are actually kcal)
CALCULATIONS
Note: s = 1.0 cal/goC; 1 cal = 4.184 J, 1 mL H2O = 1 g H2O
Mass of Water in Calorimeter
Temperature Change (DT ) of Water
Energy (Q) absorbed by the calorimeter (Help)
In calories:
In Joules:
Energy content (J/g) of the food
Calculate the energy content (J/g) that would be expected, based upon the data given on the food package. Compare this with the energy content that you measured in the lab. Comment on the variations you observe. (Help)
|
1 serving |
kcal |
cal |
J |
|
g |
serving |
kcal |
cal |