Gravimetric Analysis of a Sulfate
Mixture
Objective
To determine the relative
percentages of sodium and potassium sulfates in an unknown mixture
Concepts
Gravimetric analysis, stoichiometry, mole ratios
Introduction
In this experiment you will
be given a sample that contains sulfate ion. It is a mixture of two compounds,
sodium sulfate and potassium sulfate, both anhydrous salts. Your first task is
to determine the total number of moles of sulfate ion in the original mixture.
From that quantity and using the total mass of your sample and the molar masses
of the two components of the mixture, you will be able to determine the
composition of the sulfate mixture.
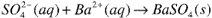
The precipitate is collected
by centrifugation, washed carefully, dried, and weighed. This experiment is an
example of gravimetric analysis. You can expect to spend about an hour on
the first day, plus short periods of time on one or more successive days,
perhaps while another experiment is in progress.
Prelaboratory
Assignment
1. Read the entire experiment before coming
to the laboratory.
2. Prepare a data table for recording
masses and descriptive observations.
Prelaboratory
Questions (click here for help)
1. Write the balanced molecular and
complete-ionic equation for the reaction between barium chloride and potassium
sulfate in aqueous solution to form barium sulfate. Show that the net-ionic
equation for this reaction is the same as was given above.
2. Suggest a reason why it is desirable to
use an excess of barium ion in the precipitation.
3. A sample of an unknown sulfate compound
has a mass of 0.1000g. Addition of excess barium chloride
solution to the sample forms a barium sulfate precipitate of mass
0.0676g. What is the mass percent of sulfate ion in the unknown compound?
4. Hydrated calcium sulfate, CaS04,
contains of 55.8 sulfate ion, by mass. Calculate the number of waters of
hydration that are present in the hydrate. Write the correct formula for the
hydrate.
Safety Precautions
1. Chemical splash-protective eyewear must
be worn at all times in the laboratory.
2. Hydrochloric acid is corrosive to skin
and clothing.
3. Barium compounds are toxic. Wash
thoroughly with soap and water before leaving the laboratory.
Materials
Procedure
For the most precise results, duplicate determinations should be carried
out, each following the sequence of steps given below.
The sample mass should be in the range of 100-150 mg if a balance with
milligram sensitivity is used, or 50-100 mg if an analytical balance is
available.
Note: If you were given a numbered unknown sample, be sure
to record the number in your notebook.
1. Determine the mass of a clean, dry
100-mm test tube. Place a small amount of the unknown
(about the volume equivalent of one grain of rice) in the tube, and then
reweigh the tube and contents. Add about
1 mL of distilled water to dissolve the sample, gently warming the tube on a
hot plate or carefully in a burner flame if necessary. If the mixture is
heated too strongly, it may "bump" and some of the liquid may be
lost. Some solids may require more water, but be careful not to fill the tube
beyond about 1/3 full. The sample must be completely dissolved before
proceeding. Add 2 drops of 1 M HCl to the tube and shake gently. Follow this with 0.5-1.0
mL of 1.0 M BaCl2 added dropwise, with gentle shaking
after each 2-3 drops. Take care not to get barium chloride on your skin; if you
do, wash it off with soap and water. Warm the tube and contents on a hotplate
for 2-3 minutes to aid coagulation of the precipitate. Do not boil.
2. Remove the tube from the heat, allow it
to cool, then centrifuge for 30 seconds. Without disturbing the solid on the
bottom of the test tube, add one more drop of the barium chloride solution. If
no new cloudiness appears, proceed with the washing of the precipitate (step
4). If cloudiness is observed, add five more drops of the BaCl2,
then heat, centrifuge, and test again with barium chloride; continue in this
fashion until addition of barium chloride does not cause further cloudiness.
3. Decant and discard the clear supernatant
solution above the barium sulfate precipitate, being careful not to lose any
solid. A microtip transfer pipet or Pasteur pipet is
useful for this purpose. Add about 10 drops of ice-cold distilled water to the
solid in the tube, then shake the tube and contents
until all of the precipitate is suspended in the water (it will not dissolve).
Centrifuge the suspension and again discard the clear, colorless supernatant,
being careful not to lose any of the white solid. Repeat the ice-water rinse,
centrifuging, and decanting twice more, followed by a final rinsing with
acetone.
4. Dry the test tube and precipitate first
on a steam bath to drive off most of the acetone and then in a 100-110°C oven
for at least one hour, preferably overnight.
5. Remove the tube from the oven and allow
it to cool for a minimum of 30 minutes in a desiccator. Determine the mass of the tube and contents.
Return the tube to the oven for at least an hour, cool in the desiccator once
more, then reweigh it. If the mass agrees within
experimental error (± 0.5 of the precipitate mass) with the previous value, the
experiment is completed. If not, continue the cycle of oven-drying, cooling,
and weighing until a constant mass is obtained.
Disposal
1. Barium sulfate, like barium chloride, is
toxic by ingestion. Transfer your solid product to an appropriate container for
removal as hazardous waste. Small amounts of barium ion were washed into the
effluent stream during the decanting and rinsing portions of the procedure;
this is unavoidable, but the amounts are below the parts-per-trillion level, so
present no significant hazard to the environment.
2. Wash all glassware immediately after use
and return it to its proper location.
Processing
the Data (click
here for help)
1. Determine each of the following, showing
appropriate calculations to support your results. If you did two or more
trials, as was suggested, you need only show calculations for one of the
trials, with results for all presented in the form of a table, with separate
columns for each sample.
· Mass of original sulfate sample
· Mass and moles of barium sulfate
produced
· Moles and mass of sulfate ion present in
unknown sample
· Mass percent of sulfate ion in unknown
2. The unknown that you used was a mixture
of potassium sulfate, K2S04, and sodium sulfate, Na2S04.
Here's what you know:
· The total number of moles of barium
sulfate equals the number of moles of sulfate ion in the original sample,
which, in turn, must equal the combined moles of K2S04
and Na2S04.
· The total mass of the original sample
must be the combined masses of K2S04 and Na2S04.
· The mass of K2S04
is found by multiplying the number of moles of K2S04 by
the molar mass of K2S04; likewise, the mass of Na2S04
is found by multiplying the number of moles of Na2S04 by
the molar mass of Na2S04.
· The number moles of Na2S04
must be the difference between the total moles of sulfate and the number of
moles of K2S04 in the original sample.
Given this information it is a simple matter of algebra to determine the
masses of K2S04 and Na2S04 that
were in the original sample, and from those you can establish the mass percent
composition of the sample mixture. Thus, if the number of moles of barium
sulfate recovered is, say, 7.50 x 10-4 mol, and if the number of
moles of K2S04 is represented by x, then the number of moles of Na2S04
would be (7.50 x 10-4 -x). If you carried out multiple
trials, you need only show the actual calculations for one trial; simply report
the masses ofK2S04 and Na2S04, and
the percentage composition of the mixture for each additional trial.
Analysis
and Conclusions (click
here for help)
1. Suggest explanations for each of the
following parts of the procedure.
a. The first three rinsings
of the product specified the use of distilled water that was ice-cold, rather
than room temperature.
b. Acetone is used for the final rinse.
2. What would be the effect on your
determination of the mass of barium sulfate in each of the following cases? For
each one, you are to decide whether the reported mass ofBaS04 would be too
high, too low, or unaffected. Explain the reasoning behind your choice.
a. Too little barium chloride solution was
used.
b. The sample was not thoroughly dried.
c. The tube and contents were not cool
before the final weighing.
3. The purpose of adding hydrochloric acid
in Part B is to remove any carbonate ions that might be present in the unknown,
so that barium carbonate will not precipitate along with the sulfate. Write the
net-ionic equation for the reaction between protons (hydrogen ions) in solution
and dissolved carbonate ions. What effect on your sulfate percentage could
result if the addition of acid were omitted? Explain.
4. What effect on your sulfate percentage
could result if the addition of acid were omitted? Explain.
5. Error analysis: Discuss experimental errors and identify
those portions of the procedure where extra care is needed to ensure
satisfactory results.
![]()