Density
of water.
The object of this portion of the lab is obviously to determine the density of water. Remember that density is calculated by dividing mass by volume.
d = m/V where d is density, m is mass and V is volume.
One would figure that all one needs to do is weigh an amount of water, measure its volume and then divide the two quantities, but since this is chemistry class we are going to do it the hard way.
You are going to prove that your measurements together will give you the density of water by way of constructing a graph. Look at your completed table on your lab, you will see that the masses of water are increasing. What needs to be determined is if the masses of water are increasing at the same rate as the volumes of water. Note that the volumes of water are measured in mL (milliliters) Remember that 1 mL = 1 cm3 so whether you are measuring volume using a graduated cylinder or a ruler a volume should easily be measured.
Constructing your graph:
In plotting your points use only the volumes in the first column and the masses of water in the third column. The masses in the third column exclude the mass of the graduated cylinder.
Be sure to use a ruler in constructing your graph and take up at least a ½ sheet of paper. In this particular graph plot the volumes on the x-axis (horizontal line) and the masses on the y-axis (vertical line)
Use straight edges. Freehanded line will not be accepted
Plotting the best-fit line and determining slope.
To plot a best fit line use a ruler to construct a straight line through the plots covering as many plot as possible.
For example:
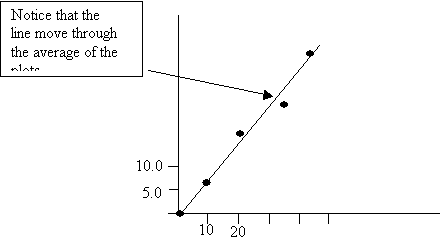
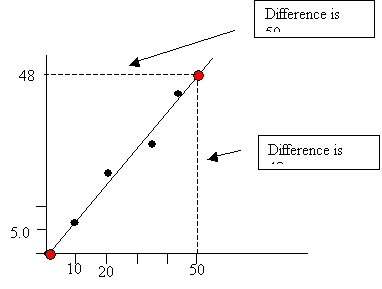
To calculate slope pick two points on the line and determine the difference in there displacement. By dividing the difference in the two chosen points slope is calculated. In this example the difference the plots rose was 48 and the difference the plots ran across is 50. Therefore, if you divide rise over run or 48/50 a slope of 0.96 is determined, and slope equals density. So the density for this particular graph is 0.96 g/mL.