Redox Titration Lab
Purpose
To
determine the percent by mass of iron in the given sample by using the
titration method
Introduction
Reduction/oxidation
(redox) processes occur when electrons are transferred
from a donor species (the reducing agent) to another acceptor species (the
oxidizing agent). The loss of electrons is oxidation; the gain of electrons is
reduction. Transfer of electrons can be illustrated by the following simple
example:
Zn(s) + Cu2+ (aq) → Zn2+ (aq)
+ Cu(s)
The plating of solid copper from solution
on a zinc surface is actually two chemical processes, complementary to each
other and occurring simultaneously:
Zn(s) → Zn2+
(aq) + 2e- (oxidation)
Cu2+(aq) + 2e- → Cu(s)
(reduction)
The zinc
metal loses electrons (is oxidized) to become aqueous zinc ion, while the
copper ions gain electrons (are reduced) to become uncharged copper metal
atoms. Equations that describe only the oxidation or reduction process are
called half-reactions. Half- reactions are a useful means of balancing redox equations.
In this
experiment you will use a redox reaction between iron
(II) ions and permanganate (MnO4-) ions to determine the
percent iron by mass in an unknown sample. The reaction can be partially
described by the BALANCED equation shown below:
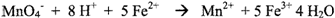
The H+ indicates that
this reaction occurs under acidic conditions. In this reaction, electrons are transferred
from the iron (II) ion to the permanganate ion, liberating manganese (II) ion
and oxidizing the iron (II) to iron (III). The permanganate ion is deeply
colored (purple); the manganese (II) ion is colorless. As permanganate is added,
more iron (II) ion is consumed, generating more manganese (II) ion and causing
the color of the permanganate to disappear. When all the iron (II) is oxidized
to iron (III), any additional permanganate will impart a permanent color to the
solution because it will have nothing to react with. This is the end-point of
the titration.
The
potassium permanganate is standardized against sodium oxalate in acid solution:
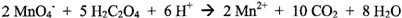
Potassium
permanganate acts as its own indicator both in the standardization and in the
iron determination,
Pre-lab
Questions (click here for help)
1.
How
many moles of Fe ion can be oxidized by 0.012 moles MnO4-
ion in the reaction? Show work.
2.
A
solid sample containing some Fe2+ ion weighs 1.923 g. It requires
36.44 mL of 0.0244 M KMnO4 to titrate the Fe2+ in the
dissolved sample to a pink end point. Calculate the grams of iron in the sample
and the percentage of iron in the sample. Show work with units.
3.
What
is the percentage of iron in iron (II) ammonium sulfate hexahydrate?
Show work.
(Hint - this is obtained from the formula of the compound.)
Procedure
A.
Preparation of the potassium permanganate
solution
Prepare
250 mL of 0.1 M KMn04 solution in a 250 mL standard flask.
B.
Preparation of the sodium oxalate solution
Prepare
100 mL of 0.25 M Na2C204 solution in a 100 mL
standard flask.
NOTE:
1. The
potassium permanganate solution must be used when freshly made to obtain
accurate results. Therefore, this entire experiment must be completed on the
same day.
2. Add the
permanganate directly into the oxalate solution (not down the walls ofthe beaker). Promptly wash down any KMn04 that spatters
on the walls of the beaker into the bulk of the liquid using a wash bottle.
3. Finely
divided MnO2 will form if the KMnO4 is added too rapidly
and will cause the solution to acquire a faint brown discoloring. This is not a
serious problem of sufficient oxylate remains to
reduce the MnO2 to Mn2+; simply discontinue the titration
until the brown color disappears.
4. The
surface of the permanganate solution rather that the bottom of the meniscus can
be used to measure titrant volumes.
5. Partial
decomposition of the permanganate to MnO2 may occur if it remains in
the buret for a long time. Clean the buret with a dilute sodium bisulfite
solution.
C.
Standardization of potassium permanganate
solution with sodium oxalate solution
Pipet out
20 mL of 0.25 M sodium oxalate solution into an Erlenmeyer flask. Add 15 mL of
3 M H2S04 to the flask and heat to about 80 - 90°C. Titrate the hot solution with potassium permanganate while stirring
with a magnetic stirrer. The pink color imparted by one addition should be
permitted to disappear before further titrant is
added. The temperature should not drop below 60°C. The end point is marked by
the appearance of a faint pink color that persists at least 30 seconds.
(Make your
Data table)
D.
Analysis of the give iron compound by
titration using the standardized potassium permanganate solution
You will
get an unknown iron sample from your instructor. Weigh out 0.2 g into a 250 mL
Erlenmeyer flask. Dissolve the sample by adding about 15 mL of deionized water
and then add 15 mL of 3M sulfuric acid. Titrate the
unknown samples as you did in part C. (You need not heat the solution). Stop
titrating when a permanent pale pink color appears. Record initial and final buret readings for each sample.
(Make
your Data table)
Calculations:
(Click here for help)
1.
Calculate
the molarity of the permanganate solution using the
standard sodium oxalate solutions molarity and the titer value.
2.
Moles
of permanganate ion used in part D =
3.
Moles
of iron ion present in the sample =
4.
Mass
of iron ion present in the sample =
5.
Fe
present in the sample =
Post
lab Questions:
1.
Discuss
three sources of error and improvements to the errors that you discuss.
2.
How
would these actions affect your calculated value of the moles of the iron?
a.
When
you finished a titration and had recorded your data, you noticed small drops of
the solution still clinging to spots on the inside walls of the buret near the top of the buret.
b.
You
added the KMnO4 rapidly at the beginning and the solution had a
slightly muddy brown color that would not go away. Since it was not very dark,
you continued the titration to the faint pink end point.
c.
Potassium
permanganate will slowly decompose in strong sunlight to MnO2 and is
usually stored in dark glass bottles. You standardized your KMnO4
one day, and stored it in a clear bottle on the window sill. Several days later
you used this solution to determine the iron.
d.
When
you were adding drops of KMnO4 solution to the Erlenmeyer flask, a
drop of the solution fell in after you reached the end point.
e.
If
you did not add the H2SO4 solution to one of the wells
but added H2O. During the titration the solution turned muddy brown.
3.
What
chemistry themes apply to this lab?
![]()
![]()